Corrosion generally occurs when most or all of the atoms on the same metal surface are oxidized, damaging the entire surface. Most metals are easily oxidized: they tend to lose electrons to oxygen in the air or in water. As oxygen is reduced, it forms an oxide with the metal. It is a process through which metals in manufactured states return to their natural oxidation states. This process is a reduction-oxidation reaction in which the metal is being oxidized by its surroundings, often the oxygen in air. This reaction is both spontaneous and electrochemically favored. Corrosion is essentially the creation of voltaic, or galvanic, cells where the metal in question acts as an anode and generally deteriorates or loses functional stability.
Corrosion is one of the highest concerns for industrial people. It affects steel as well as concrete surfaces under the abuse of acids, alkalies, chemicals, solvents, etc. Nearly US$ 5,000 is annual loss occurring globally due to loss of metal on account of corrosion. Corrosion engineering experts such as Huguenot Lab. combines industry experience in testing and mitigating microbial and generalized corrosion in fire protection systems (FPS).There are different types of steel corrosion and each of them has different structural effects, each of these is explained below. Therefore it is pivotal to be aware of Corrosion Prevention Methods.
Types of Corrosion Prevention Methods
Barrier Coating for Corrosion Prevention
A barrier coating forms an insulating and physical barrier, thus stopping the contact of corrosive elements, such as the electrolyte, with the substrate. Barrier coatings are typically applied on metals and ceramics when they are unable to withstand very harsh operating conditions. Many industrial processes occur at very high temperatures under the flow of harsh, corrosive gases. Metals often can corrode, which can lead to catastrophic failure during operation. To prevent this, and to enhance their life and operating efficiencies, metals and ceramic components are coated with a different material that can withstand these demanding environments.The property of the barrier coating material has to be matched with that of the underlying substrate.
Barrier coating aims to prohibit water, oxygen and other chemicals from making contact with the substrate. In reality, it’s taken for granted that some water and oxygen will reach the surface that barrier coatings protect. But since the water that does make it through a barrier coating isn’t significantly charged (meaning there is not a heavy concentration of ions in the water), the fundamental elements necessary for kicking off the corrosion process are not all present.

Hot-dip galvanization for Corrosion Prevention
Hot-dip galvanization is a form of galvanization. It is the process of coating iron and steel with zinc, which alloys with the surface of the base metal when immersing the metal in a bath of molten zinc at a temperature of around 449 °C (840 °F). Hot dip galvanizing offers coverage both externally and internally within hollow sections, it self-repairs when damaged, sacrifices itself to protect the base metal, is environmentally sustainable, has good impact and abrasion-resistance.Like other corrosion protection systems, galvanizing protects steel by acting as a barrier between steel and the atmosphere.
Hot-dip galvanizing provides a number of benefits to the steel it protects. The metallurgically-bonded zinc-iron alloy layers not only create a barrier between the steel and the environment, but also cathodically protect the steel. The cathodic protection offered by zinc means the galvanized coating sacrifices itself to protect the underlying base steel from corrosion. The tightly adhered coating, which has bond strength of around 3,600 psi, is also extremely abrasion-resistant, as the intermetallic layers are harder than the base steel. However, even if the coating is damaged, zincs sacrificial action will protect exposed steel up to ¼ inch away.

Alloyed Steel (Stainless) for Corrosion Prevention
When other elements comprising metals and non-metals are added to carbon steel, alloy steel is formed. These alloy steels display various environmental, chemical and physical properties that can vary with the elements used to alloy. Here the proportion of alloying elements can provide different mechanical properties. Resistance to corrosion and staining, low maintenance, and familiar luster make stainless steel an ideal material for many applications where both the strength of steel and corrosion resistance are required. Moreover, stainless steel can be rolled into sheets, plates, bars, wire, and tubing. Alloying elements can alter carbon steel in several ways. Alloying can affect micro-structures, heat-treatment conditions and mechanical properties. Today’s technology with high-speed computers can foresee the properties and micro-structures of steel when it is cold-formed, heat treated, hot-rolled or alloyed. For instance, if properties such as high strength and weldability are required in steel for certain applications, then carbon steel alone will not serve the purpose because carbon’s inhere ..
Alloy steels are made by combining carbon steel with one or several alloying elements, such as manganese, silicon, nickel, titanium, copper, chromium and aluminum. These metals are added to produce specific properties that are not found in regular carbon steel. The elements are added in varying proportions (or combinations) making the material take on different aspects such as increased hardness, increased corrosion resistance, increased strength, improved formability (ductility); the weldability can also change.

Cathodic protection for Corrosion Prevention
Cathodic Protection is an electro-chemical process that slows or stops corrosion currents by applying DC current to a metal. When applied properly, CP stops the corrosion reaction from occurring to protect the integrity of metallic. Cathodic protection works by placing an anode or anodes (external devices) in an electrolyte to create a circuit. Current flows from the anode through the electrolyte to the surface of the structure. Corrosion moves to the anode to stop further corrosion of the structure. In essence, cathodic protection connects the base metal at risk (steel) to a sacrificial metal that corrodes in lieu of the base metal. The technique of providing cathodic protection to steel preserves the metal by providing a highly active metal that can act as an anode and provide free electrons. By introducing these free electrons, the active metal sacrifices its ions and keeps the less active steel from corroding.
There are two basic types of cathodic protection: galvanic, and impressed current cathodic protection. Galvanic protection consists of applying a protective zinc coating to the steel to prevent rusting. The zinc corrodes in place of the encapsulated steel. These systems have limited life spans, as the sacrificial anode protecting the underlying metal will continue to degrade over time until the sacrificial anode is no longer capable of supplying protection. Impressed current cathodic protection systems consist of anodes that are connected to a power source that provides a perpetual source of electrical flow. The sacrificial anode method of protection uses a metal more active than the base metal to “sacrifice” ions. These “sacrificial anodes” (usually alloys such as magnesium, aluminum, or zinc) have a stronger electrochemical potential. This method can often provide much longer protection than a sacrificial anode, as the anode is supplied by an unlimited power source.

Sacrificial Protection for Corrosion Prevention
Sacrificial protection is a corrosion protection method in which a more electrochemically active metal is electrically attached to a less active metal. The highly active metal donates electrons to replace those which may have been lost during oxidation of the protected metal. This reverts the protected metal back to its original form, and thereby prevents it from corroding. Materials used as sacrificial anodes are either purely active metals, such as magnesium or zinc, or the alloys of either aluminum or magnesium specifically developed to be used as sacrificial anodes.
The sacrificial protection may be provided by using the anodes, usually supplied with cast-m straps or lead wires. The lead wires can be attached to the structure using mechanical or welded connections, and should have low resistance and insulation to prevent them from corroding or becoming damaged mechanically.
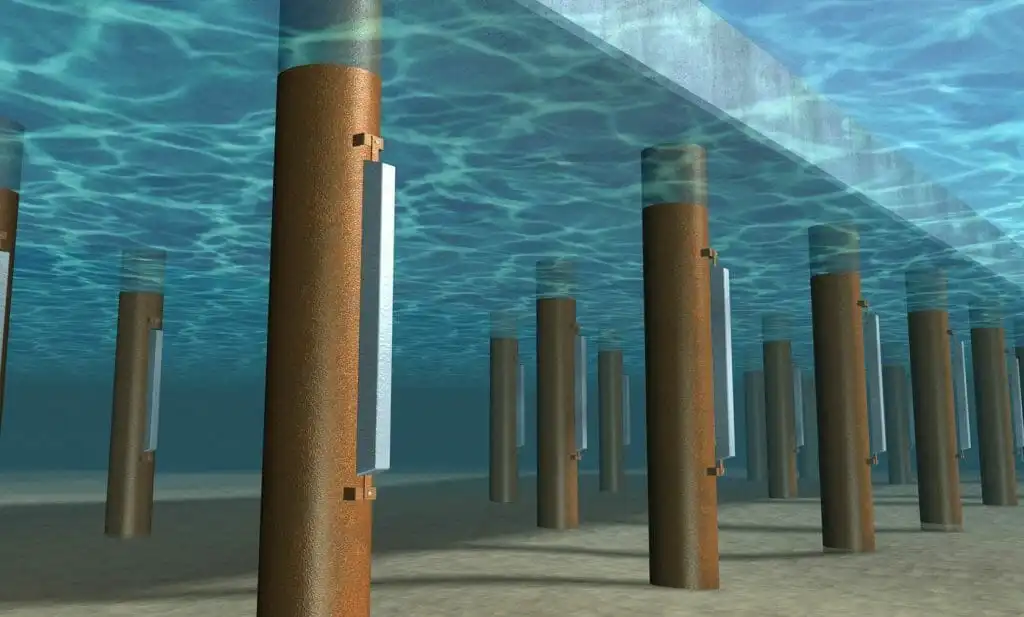
Image Source: coatingpaint.com, galco.ie, eoncoat.com, marcepinc.com, cathwell.com



